
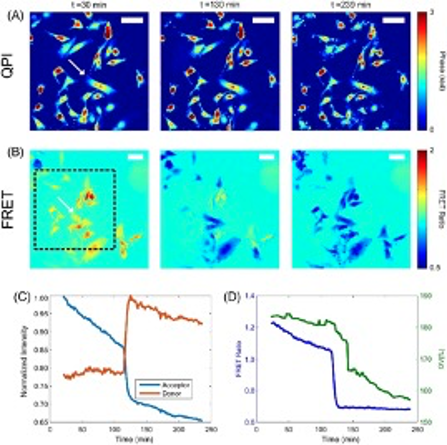
Chemical, mechanical, and electrical stimuli have all been shown to modulate the transmission state of active ion channels in cells. However, there is still a lack of understanding of how mechanical and electrical stimuli interact to produce changes in cell signaling pathways and sub-cellular structures. Figure 1 shows our custom-built microscopy system capable of performing simultaneous quantitative phase microscopy (QPM) and Förster resonance energy transfer (FRET) measurement to study the structural, mechanical, and electrical properties of cells at the nanoscale. An example of this capability is seen in Figure 2, where we are tracking the optical volume and FRET signal of cells undergoing apoptosis. FRET imaging can be exploited to assess local tension through nanoscale measurements of force transduction by specific molecules within cell structures. FRET sensors can also be utilized to detect both membrane potential and concentration of calcium ions to relate mechanical information to channel activation. Integration of QPM allows for visualization of cell structure with nanometer depth sensitivity and at millisecond time scales. Previously, we developed QPM methods to use this nanoscale information to distinguish cell phenotype based on mechanical properties, track stiffness changes during carcinogenesis, and estimate shear moduli from our QPM shear flow assay. We seek to further develop this QPM toolbox and use it in conjunction with FRET to study how external stimuli are transduced by cellular structures to produce changes in the internal electrical state via modulation of ion channel transmission. Fig 2. from Eldridge et. al. 2018.